Background: Secondary or treatment-related acute myeloid leukemia (sAML) is associated with poor outcomes. Although allogeneic hematopoietic cell transplantation (alloHCT) is the treatment of choice, patient eligibility criteria and optimal conditioning regimen remain under study. We have previously shown an advantage of a myeloablative conditioning regimen with reduced toxicity (Fludarabine 150mg/m2, Treosulfan 42g/m2, FluTreo) compared to a reduced intensity regimen. However, the long-term effects of this regimen remain unknown, especially in comparison to patients not receiving alloHCT.
Aims: We hypothesized that patients transplanted with FluTreo would have a long-term survival advantage over other treatment alternatives.
Methods: We retrospectively studied consecutive patients treated for sAML in our center over the last two decades (1998-2018). Exclusion criteria were: age>70 years, ECOG performance status≥3 at presentation, induction regimens≤2, and autologous or haploidentical HCT because these were performed in individual patients. Since 2013, we have introduced FluTreo for patients with a suitable donor that would have been previously eligible only for reduced intensity conditioning/RIC. The following factors were studied in the whole cohort: age, type of disease (treatment-related or post myelodysplastic syndrome/MDS), previous intensive chemotherapy cycles, cytogenetic risk, overall (OS) and disease-free survival (DFS). In transplant recipients, we also recorded HCT-comorbidity index/CI score, cumulative incidence (CI) of graft-versus-host disease (GVHD), and treatment-related mortality (TRM). Follow-up was calculated from diagnosis in the whole cohort; and from date of transplant in the sub-group analysis of transplant recipients.
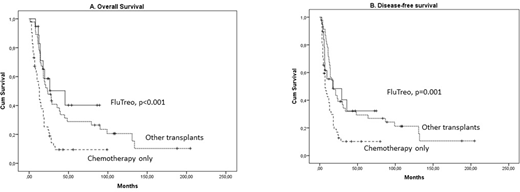
Results: We studied 19 FluTreo recipients compared to 46 recipients of other conditioning regimens (38 myeloablative and 8 RIC), and 52 patients treated only with chemotherapy. Complete remission (CR) had been achieved in all FluTreo recipients before HCT, compared to 53% of other transplants, and 44% of chemotherapy only patients (p<0.001). As expected, median age was increased in FluTreo and chemotherapy only patients (59 and 58 years respectively), compared to other transplants (48 years, p<0.001). There was no other difference in baseline characteristics. With a median follow-up of 43 (range 13-204) months in surviving patients, 4-year OS was 40.3% in FluTreo versus 31.3% in other transplants and 11.8% in chemotherapy only patients (p<0.001, Figure 1A). In the multivariate analysis, achieving CR (p<0.001) and the FluTreo group (p<0.001) were associated with favorable OS, independently of age and cytogenetic risk. 4-year DFS was 32.3% in FluTreo, versus 29.3% in other transplants and 10.2% in chemotherapy only patients (p=0.001, Figure 1B).
Within transplanted patients, there was no significant difference in GVHD, relapse and TRM between FluTreo and other transplants. Within the FluTreo group, acceptable rates of CI in severe acute and extensive chronic GVHD were observed (15.2% and 18.4%, respectively). Similarly, 4-year CI of TRM reached 30.4%, despite a median HCT-CI of 3 (0-7), age of 59 (51-67) years, 4 lines of treatment (3-6), and a majority of matched unrelated donors (13/19).
Conclusion: Our real-world study confirms that alloHCT with FluTreo expands the transplant population with similar safety and efficacy to previous transplant modalities in sAML patients. Achievement of CR remains a major predictor of OS in these patients. The choice of alloHCT in this unique patient population of a rather older age and comorbidity index needs to be revisited.
Gavriilaki:Omeros Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal